Background. Although several new treatments are available for patients with multiple myeloma (MM), most patients eventually relapse at a median time of 8.0 months (95%CI: 6.3-8.9). Patients with relapsed and refractory MM (R/R MM) who have had several lines of previous therapy or who are refractory to lenalidomide and proteasome inhibitors require alternative options. Daratumumab and isatuximab are monoclonal antibodies that bind to the human CD38 receptor. Phase II/III clinical trials showed that isatuximab (ISA) or daratumumab (DARA) in combination with pomalidomide (POM-d) and low-dose dexamethasone (DEXA) significantly improve progression-free survival (PFS) in patients with R/R MM. No studies have assessed the comparative efficacy and cost-effectiveness of both regimens in management of R/R MM. We performed an indirect comparison of both regimens in terms of PFS and overall survival (OS) and evaluated the cost-effectiveness and cost-utility of DARA+POM-d+DEXA and ISA+POM-d+DEXA from a US payer's perspective.
Methods. A partitioned survival model was developed to create three health states (pre-progression, progression, and death). The model was run three times with different time horizons (one, three and five years). To simulate health outcomes for each treatment regimen, transition probabilities between the three health states were derived from parametric exponential and lognormal distributions fitted to Kaplan-Meier (KM) curves of PFS and OS of the phase Ib clinical trial (Chari et al.; Blood 2017) for DARA+POM-d+DEXA and the phase III clinical trial (Attal et al.; Lancet 2019) for ISA+POM-d+DEXA. Wholesale acquisition costs (WAC) were obtained from RedBook for each regimen. Pre-progression costs included costs of regimens; premedication (50 mg diphenhydramine, 650 mg acetaminophen, 50 mg ranitidine); managing side effects; routine care and monitoring; and medication administration. Costs were inflated based on the medical consumer price index to the second quarter of 2020. Utilities were obtained from literature and assumed the same for both interventions. Annual discount rate of 3.5% was applied for costs and outcomes beyond the first year. The life years (LY) and quality adjusted LY (QALY) for each treatment, and the incremental cost-effectiveness (ICER) and cost-utility ratios (ICUR) were estimated in both base and probabilistic sensitivity analyses (PSA:10,000 simulations). The cost-effectiveness plane (CEP) and cost-effectiveness acceptability curves (CEAC) were plotted.
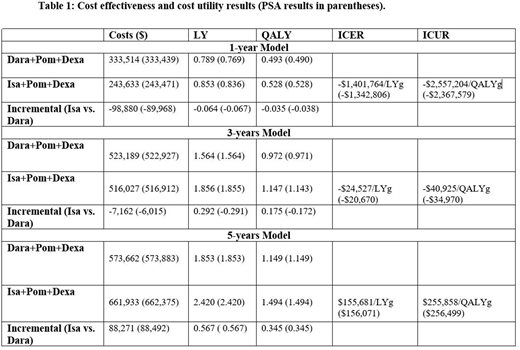
Results. In the naïve patient simulation, median PFS and OS were estimated to be 9.5 months and 18 months for DARA+POM-d+DEXA, and 14.5 months and 26 months for ISA+POM-d +DEXA. As shown in the table below, ISA+POM-d+DEXA is associated with greater LY and QALY gains at one-, three- and five-year time horizons. The costs of ISA+POM-d+DEXA at one- and three- year time horizons are less than that of DARA+POM-d+DEXA, which resulted in saving (decremental) ICERs. At 5 years' time horizon, ISA+POM-d+DEXA was associated with incremental benefits (0.57 LY, 0.35 QALY) and incremental costs of $88,271 when compared with DARA+POM-d+DEXA. Per the CEAC plot, the probability that ISA+POM-d+DEXA is cost-effective was 100%, 65% and 23% at a willingness to pay threshold (WTP) of $100,000 per QALY in one-, three- and five-year time horizons.
Conclusions. Clinically, ISA+POM-d+DEXA is associated with incremental survival gains of ~1 month and quality-adjusted survival gains of 0.5 month than DARA+POM-d+DEXA when patients are treated for one year. The benefits increase with treatment duration to reach ~7 months life year gains and 4 months quality-adjusted life year gains if patients treated for 5 years. Due to its lower total costs, Isatuximab based-regimen yielded saving ICERs at one and three years. However, ISA+POM-d+DEXA cost exceeded DARA+POM-d+DEXA at 5 years' time horizon to yield an ICER above the WTP.
McBride:Merck: Speakers Bureau; Pfizer: Consultancy; Sandoz: Consultancy; MorphoSys: Consultancy; Bristol-Myers Squibb: Consultancy; Coherus BioSciences: Consultancy, Speakers Bureau. Abraham:Celgene: Consultancy; Terumo: Consultancy; Rockwell Medical: Consultancy; Janssen: Consultancy; Mylan: Consultancy; Sandoz: Consultancy; Coherus BioSciences: Research Funding, Speakers Bureau; MorphoSys: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.